CHAPTER 10 STATES OF MATTER
CHAPTER 10 STATES OF MATTER. Sections. 10.1 – Kinetic Molecular Theory 10.2 – Liquids 10.3 – Solids 10.4 – Changes of State 10.4 – Water. 10. 1 Kinetic Molecular Theory. State the kinetic-molecular theory of matter, and describe how it explains certain properties of matter.
Share Presentation
Embed Code
Link
Download Presentation
- capillary action video
- crystal symmetry
- high pressures
- different gases
- high melting points
- covalent compounds

kent + Follow
Download Presentation
CHAPTER 10 STATES OF MATTER
An Image/Link below is provided (as is) to download presentation Download Policy: Content on the Website is provided to you AS IS for your information and personal use and may not be sold / licensed / shared on other websites without getting consent from its author. Content is provided to you AS IS for your information and personal use only. Download presentation by click this link. While downloading, if for some reason you are not able to download a presentation, the publisher may have deleted the file from their server. During download, if you can't get a presentation, the file might be deleted by the publisher.
Presentation Transcript
- CHAPTER 10STATES OF MATTER
- Sections • 10.1 – Kinetic Molecular Theory • 10.2 – Liquids • 10.3 – Solids • 10.4 – Changes of State • 10.4 – Water
- 10. 1 Kinetic Molecular Theory • State the kinetic-molecular theory of matter, and describe how it explains certain properties of matter. • List the five assumptions of the kinetic-molecular theory of gases. • Define the terms ideal gas and real gas. • Describe each of the following characteristic properties of gases: expansion, density, fluidity, compressibility, diffusion, and effusion. • Describe the conditions under which a real gas deviates from “ideal” behavior.
- What is the Kinetic Molecular Theory? • Break it down: • Kinetic: movement • Molecular: particles • Theory: tested ideas Tested ideas about the movement of particles! This theory is used to explain the energy and forces that cause the properties of solids, liquids, and gases.
- KMT of Gases • Ideal gas: hypothetical gas based on the following five assumptions… • Gases consist of large numbers of tiny particles that are far apart relative to their size. • Most of the volume is empty space • Collisions between gas particles and between particles and container walls are elastic collisions. • elastic collisionwhen there is no net loss of total kinetic energy
- KMT cont. • Gas particles are in continuous, rapid, random motion. They therefore possess kinetic energy, which is energy of motion. • There are no forces of attraction between gas particles. • The temperature of a gas depends on the average kinetic energy of the particles of the gas. • The kinetic energy of any moving object is given by the following equation:
- Gas Behavior • KMT applies only to ideal gasses. • Most gasses behave ideally if pressure is not too high or temperature is not too low.
- Expansion • Gases: do not have a definite shape or a definite volume. Gas particles move rapidly in all directions (#3) without significant attraction between them (#4).
- Fluidity • Because the attractive forces between gas particles are insignificant (#4), gas particles glide easily past one another. • Because liquids and gases flow, they are both referred to as fluids.
- Low Density • The density of a gas at atmospheric pressure is about 1/1000 the density of the same substance in the liquid or solid state. • The reason is that the particles are so much farther apart in the gaseous state (#1).
- Compressibility • During compression, the gas particles, which are initially very far apart (#1), are crowded closer together.
- Diffusion • Gases spread out and mix with one another, even without being stirred. • Spontaneous mixing of the particles of two substances caused by their random motion: diffusion. • The random and continuous motion of the gas molecules (#3) carries them throughout the available space.
- Effusion • Effusion: when gas particles pass through a tiny opening • The rates of effusion of different gases are directly proportional to the velocities of their particles. • Molecules of low mass effuse faster than molecules of high mass.
- Diffusion vs. Effusion Video
- A Real Gas • Real gas: does not behave completely according to the assumptions of the kinetic-molecular theory. • Because particles of gases occupy space and exert attractive forces on each other, all real gases deviate to some degree from ideal gas behavior. • The more polar the molecules of a gas are, themore the gas will deviate from ideal gas behavior. • Conditions for a real gas: high pressures and low temperatures
- 10.2 Liquids • Describe the motion of particles in liquids and the properties of liquids according to the kinetic-molecular theory. • Discuss the process by which liquids can change into a gas. Definevaporization. • Discuss the process by which liquids can change into a solid. Definefreezing.
- KMT of Liquids • Liquids: definite volume and no definite shape • What does this mean about the energy in liquids compared to gases? • The attractive forces between particles in a liquid are more effective than those between particles in a gas. • This is due to intermolecular forces: • dipole-dipole forces • hydrogen bonding • London dispersion forces
- Fluidity • fluid: a substance that can flow and therefore take the shape of its container. • The particles in a liquid are not bound together in fixed positions. Instead, they move about constantly and slide past each other.
- Density and Compressibility • At normal atmospheric pressure… most LIQUIDS are a hundreds times DENSER than in a gaseous state. • LIQUIDS are much LESSCOMPRESSIBLE than GASES because liquid particles are more closely packed together.
- Diffusion • Any liquid gradually diffuses throughout any other liquid in which it can dissolve. • Because the particles are in constant motion. • Diffusion is much slower in liquids than in gases. • Liquids are more tightly packed. • Intermolecular forces slow liquid movement. • As the temperature increases, diffusion occurs more rapidly.
- Diffusion of Dye through Water
- Surface Tension • surface tension:a force that pulls adjacent parts of a liquid’s surface together, decreasing surface area to the smallest possible size (cohesion)
- The higher the intermolecular forces between the particles of a liquid, the higher the surface tension. • The molecules at the surface of the water can form hydrogen bonds with the other water, but not with the molecules in the air above
- Surface Tension Video
- Adhesion • Capillary action: attraction of the surface of a liquid to the surface of a solid (adhesion) • This attraction pulls the liquid molecules upward along the surface against the pull of gravity. • This causes the concave liquid surface, called a meniscus, that forms in a test tube or graduated cylinder
- Capillary Action Video
- Phase Change to a Gas • Vaporization: process by which a liquid or solid changes to a gas • Evaporation: when particles escape from the surface of a non-boiling liquid and enter the gas state. • Boilingchange of a liquid to bubbles of vapor that appear throughout the liquid. • Evaporation occurs because the particles of a liquid have different kinetic energies.
- Phase Change to a Solid • When a liquid is cooled, the average energy of its particles decreases. • Freezing or solidification: physical change of a liquid to a solid by removal of energy as heat
- Phase Change Video
- 10.3 Solids • Describe the motion of particles in solids and the properties of solids according to the kinetic-molecular theory. • Distinguish between the two types of solids. • Describe the different types of crystal symmetry. • Define crystal structure and unit cell.
- KMT of Solids • Solids: have definite shape and definite volume • The particles are tightly packed • All inter-particle attractions exert stronger effects in solids than in the corresponding liquids or gases. • Attractive forces hold the particles in relatively fixed positions. • Solids are more ordered than liquids and are much more ordered than gases.
- Types of Solids • crystalline solids: the most common type of solid made of crystals • crystal: substance in which the particles are arranged in an orderly, geometric, repeating pattern. • amorphous solid: when the particles are arranged randomly. Crystalline Amorphous
- Phase Change of a Crystal • Melting: physical change of a solid to a liquid by the addition of energy as heat. • Which has a higher melting point, ionic or covalent compounds? • The melting point is when… the kinetic energies of the particles within the solid overcome the attractive forces holding them together. • Sublimation: change from solid directly to a gas
- Phase Change of Amorphous Solid • Amorphous solids have no definite melting point. • example: glass and plastics • Amorphous solids are sometimes classified as supercooled liquids,which are substances that retain certain liquid properties even at temperatures at which they appear to be solid. • These properties exist because of the arrangement of amorphous solids.
- Properties of Solids • Substances are usuallymost dense in the solid state. • Why are solids usually the most dense? • Why is water an exception? • Solids can be considered incompressible. • Solids diffuse millions of times slower than liquids.
- Crystals • Solids exist as single crystals or as groups of crystals fused together. • The total three-dimensional arrangement of particles of a crystal is called a crystal structure. • The arrangement of particles in the crystal can be represented by a coordinate system called a lattice. • The smallest portion of a crystal lattice that shows the three-dimensional pattern of the entire lattice is called a unit cell.
- Unit Cells
- Ionic Crystals • Ionic crystals — positive and negative ions arranged in a regular pattern. • Generally, ionic crystals form when Group 1 or Group 2 metals combine with Group 16 or Group 17 nonmetals or nonmetallic polyatomic ions. • These crystals are hard and brittle, have high melting points, and are good insulators.
- Covalent Network Crystals • Covalent network crystals — each atom is covalently bonded to its nearest neighboring atoms. • The covalent bonding extends throughout a network that includes a very large number of atoms. Represented by a metal and a polyatomic ion. • The network solids are very hard and brittle, have high melting points and are usually nonconductors or semiconductors.
- Metallic Crystals • Metallic crystals — metal cations surrounded by a sea of delocalized valence electrons. • The electrons come from the metal atoms and belong to the crystal as a whole. • The freedom of these delocalized electrons to move throughout the crystal explains the high electric conductivity of metals.
- Covalent Molecular Crystals • Covalent molecular crystals — covalently bonded molecules held together by intermolecular forces. • Nonpolar molecules: weak London dispersion forces between molecules • Polar molecules: dispersion forces, dipole-dipole forces, and sometimes hydrogen bonding • Covalent molecular crystals have low melting points, are easily vaporized, are relatively soft, and are good insulators.
- Phase Change with Heating Curve
- 10.4 Change of State • Explain the relationship between equilibrium and changes of state. • Interpret phase diagrams. • Explain what is meant by equilibrium vapor pressure. • Describe the processes of boiling, freezing, melting, and sublimation.
- Phase Changes
- Key Words • Phase: part of a system that has uniform composition and properties. • Condensation:gas changes to a liquid. • Vapor: gas in contact with its liquid or solid phase
- Equilibrium • Equilibrium: condition in which two opposing changes occur at equal rates in a closed system. • For example: In a closed system, the rate of condensation equals the rate of evaporation, and a state of equilibrium is established.
- Equilibrium of Liquid and Vapor
- Vapor Pressure • equilibrium vapor pressure: pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature • The equilibrium vapor pressure increases with increasing temperature. • Increasing the temperature of a liquid increases the average kinetic energy of the liquid’s molecules. • Every liquid has a specific equilibrium vapor pressure at a given temperature.
- Equilibrium Vapor Pressure
- Equilibrium Vapor Pressure Video Load More .

Chapter 10 States of Matter
Chapter 10 States of Matter. Section 10.1 The Nature of Gases. OBJECTIVES: Describe the motion of gas particles according to the kinetic theory. Section 10.1 The Nature of Gases. OBJECTIVES: Interpret gas pressure in terms of kinetic theory. Section 10.1 The Nature of Gases.
818 views • 64 slides

Chapter 10 States of Matter
Kinetic Molecular Theory. Is based on the idea that particles of matter are always in motion. -this theory provides a model for IDEAL gasesIdeal gas-is an imaginary gas that perfectly fits all the assumptions of the kinetic-molecular theory . The kinetic-molecular theory is based on 5 ass
1k views • 72 slides
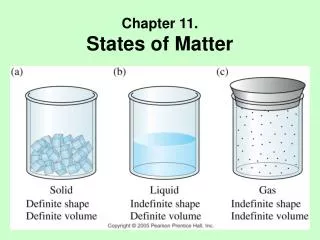
Chapter 11. States of Matter
Chapter 11. States of Matter. States of Matter. State is Determined by: Chemical Identity Temperature Pressure. States of Elements. Kinetic Molecular Theory of Matter.
10.02k views • 64 slides

Chapter 10 States of Matter
Chapter 10 States of Matter. 10.2 The nature of liquids. Things you will learn. You will understand the nature of liquids in terms of the attractive forces of particles You will be able to differentiate between evaporation of a liquid and boiling of a liquid.
517 views • 23 slides

States of Matter Chapter 13
States of Matter Chapter 13. Understand and Utilize PRESSURE of a Fluid P = Force /Area Expansion and Contraction of matter caused by changes in Temperature Ideal Gas Law PV = nRT Combined Gas Law P 1 V 1 /T 1 = P 2 V 2 /T 2 Boyles Law ( const T) P 1 V 1 = P 2 V 2
264 views • 8 slides

Chapter 13 “States of Matter”
Chapter 13 “States of Matter”. The Nature of Gases. Kinetic refers to motion The energy an object has because of it’s motion is called kinetic energy The kinetic theory states that the tiny particles in all forms of matter are in constant motion !. The Nature of Gases.
830 views • 62 slides
Chapter 6 states of matter
Chapter 6 states of matter. Name : Dimas Dwireno D Class : VII – B No. absent : 8. A. There States of Matter. classified into there states of matter which are : solid, such as iron and wood liquid, such as water and alcohol gas, such as oxygen and helium .
369 views • 19 slides

Chapter 10 States of Matter
Chapter 10 States of Matter. 10.1 The nature of gases. Things you will learn. You will understand the motion of gas particles according to the kinetic theory. You will be able to interpret gas pressure in terms of kinetic energy and absolute temperature. Gases we’re used to. O 2 CO 2
404 views • 27 slides

Ch.10 States of Matter
Ch.10 States of Matter. Mrs. Geisler Chemistry I. Kinetic Molecular Theory. Particles of matter are always in motion. Ideal gas – hypothetical gas that perfectly fits all the assumptions of the kinetic molecular theory.
402 views • 22 slides

Chapter 13 - States of Matter
Chapter 13 - States of Matter. Kinetic Theory and the Nature of Glass. All matter is composed of tiny particles in constant motion. For Gases: (PME). Made up of particles. Particles moving rapidly in constant, random motion. All collisions are perfectly elastic. (No energy lost.).
1.31k views • 107 slides

Chapter 10: States of Matter
Chapter 10: States of Matter. Concept Base: Chapter 1: Properties of Matter Chapter 2: Density Chapter 6: Covalent and Ionic Bonding. Covalent Vs. Ionic Bonding. atoms combine to form ionic bonds covalent bonds (M + NM) (NM + NM)
1.39k views • 105 slides

Chapter: States of Matter
Table of Contents. Chapter: States of Matter. Section 1: Matter. Section 2: Changes of State. Section 3: Behavior of Fluids. Matter. Everything in this photo is matter. 1. What is matter?. Matter is anything that takes up space and has mass.
959 views • 75 slides

Chapter 13 States of Matter
Chapter 13 States of Matter. In this chapter you will: Explain the expansion and contraction of matter caused by changes in temperature . Apply Pascal’s, Archimedes’, and Bernoulli’s principles in everyday situations. Chapter 13 Sections. Section 13.1: Properties of Fluids
822 views • 14 slides

Chapter 10: States of Matter
Chapter 10: States of Matter. Coach Kelsoe Chemistry Pages 328–352. Section 10–1: The Kinetic-Molecular Theory of Matter. Coach Kelsoe Chemistry Pages 329–332. Section 10–1 Objectives. State the kinetic–molecular theory of matter, and describe how it explains certain properties of matter.
402 views • 25 slides

Modern Chemistry Chapter 10 States of Matter
Modern Chemistry Chapter 10 States of Matter. Kinetic-Molecular Theory. The kinetic-molecular theory of matter is based on the idea that particles of matter are always in motion and this motion has consequences that affect its physical properties. Kinetic-Molecular Theory of Gases.
420 views • 23 slides

Chapter 13: States of Matter
Chapter 13: States of Matter. 13.1 The Nature of Gases. 13.1. Kinetic Theory and a Model for Gases. The word kinetic refers to motion. The energy an object has because of its motion is called kinetic energy.
604 views • 58 slides

Chapter 10 States of Matter
Chapter 10 States of Matter. Section 1: The Kinetic-Molecular Theory of Matter. KMT. This is based on the idea that particles of matter are always in motion . How it relates to gases: Ideal gas = a hypothetical gas that perfectly fits all the assumptions of KMT.
350 views • 30 slides

Chapter 10 States of Matter
Chapter 10 States of Matter. Essential Question. What are physical & chemical properties of liquids and solids? Standard 2h Students will identify solids and liquids held together by forces, and to relate these forces with boiling and melting points. Kinetic Molecular Theory (Ideal Gases).
228 views • 18 slides

Chapter 12: States of Matter
CHEMISTRY Matter and Change. Chapter 12: States of Matter. States of Matter. CHAPTER 12. Section 12.1 Gases Section 12.2 Forces of Attraction Section 12.3 Liquids and Solids Section 12.4 Phase Changes. Click a hyperlink to view the corresponding slides. Exit. Gases. SECTION 12.1.
1.03k views • 80 slides

Chapter 10 States of Matter
Chapter 10 States of Matter. Section 1: The Kinetic-Molecular Theory of Matter. Standard 4.b. Students know the random motion of molecules explains the diffusion of gases. We will list the five assumptions of the kinetic-molecular theory of gases and describe the properties of gases.
500 views • 40 slides






















